
Biogen and Ionis Receive the US FDA's Approval of Qalsody (tofersen) for the Treatment of Amyotrophic Lateral Sclerosis
4.9
$ 15.99
In stock
(597)
Product Description

Rise of the Genomic Medicine Era in Amyotrophic Lateral Sclerosis - Practical Neurology

FDA Expected to Decide on Tofersen Approval for SOD1-ALS in January

FDA approves QALSODY™ (tofersen) as the first treatment targeting a genetic cause of ALS

Amyotrophic Lateral Sclerosis Pipeline

amyotrophic lateral sclerosis Archives - DelveInsight
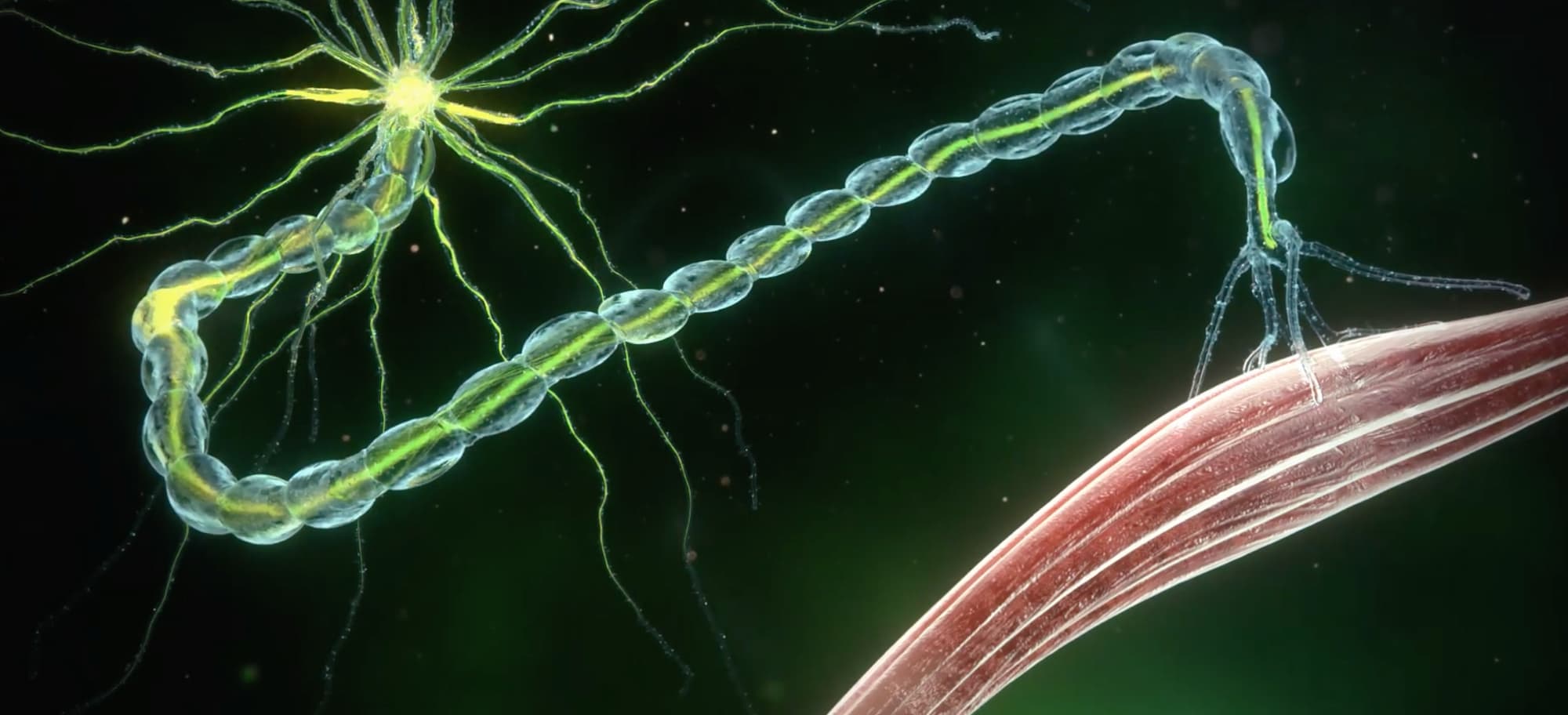
Qalsody: New Drug for Amyotrophic Lateral Sclerosis with SOD1 Mutation • BioPharma Media

Latest FDA Approvals - Biocom California

Biogen and Ionis Receive the US FDA's Approval of Qalsody (tofersen) for the Treatment of Amyotrophic Lateral Sclerosis

FDA Grants Accelerated Approval for QALSODY™ (tofersen) for

How MDA Invests in Research Success - Quest

ALS drug tofersen will face FDA advisors, says Biogen

FDA greenlights Biogen's Qalsody for rare, genetic form of ALS
How High Is the Bar for Future ALS Treatments? Although

US FDA flexibility the question as Nurown goes before adcom














